Our Products
Overview of Trefoil’s Products
The cornea accounts for 70% of the visual system’s ability to focus light. Damage through disease to corneal tissue has the potential to produce significant vision loss. These disorders affect significant patient populations. Approximately 4% of the population in the U.S. and E.U. have an endothelial disease. Ocular complications from herpes virus reactivation are the leading cause of corneal blindness with more than 4 million cases worldwide, and in the U.S. an estimated 500,000 people suffer from herpes-related eye complications.
Trefoil is advancing 2 investigational products based on TTHX1114, its engineered FGF1, to treat a spectrum of corneal diseases. Currently there are no pharmaceutical treatments for these conditions. The company’s products in development are:
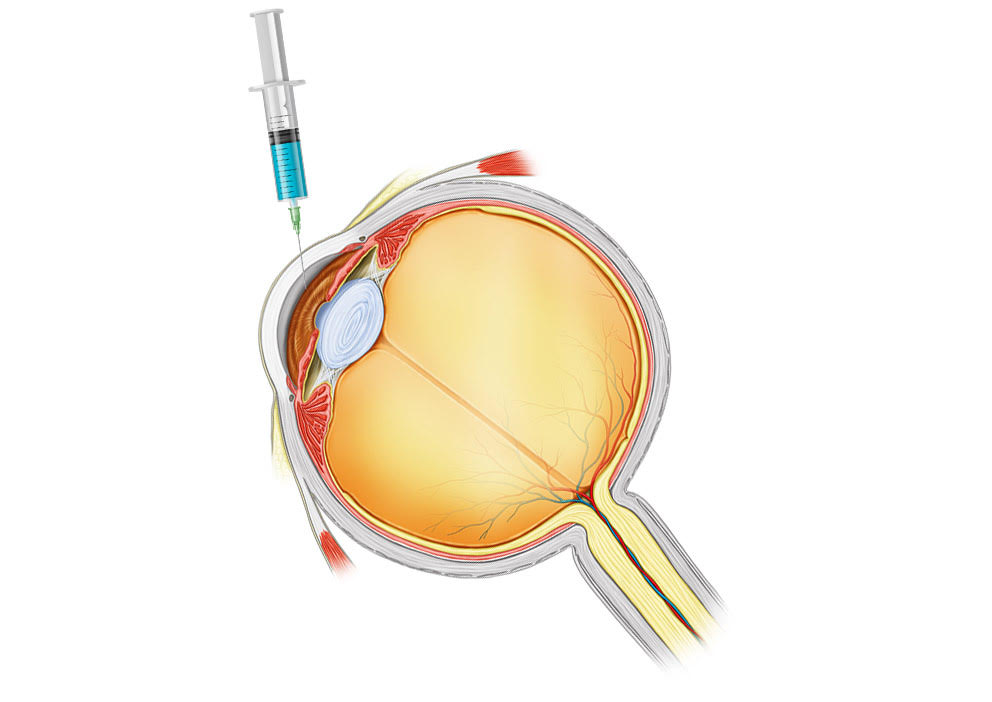
Intracameral TTHX1114
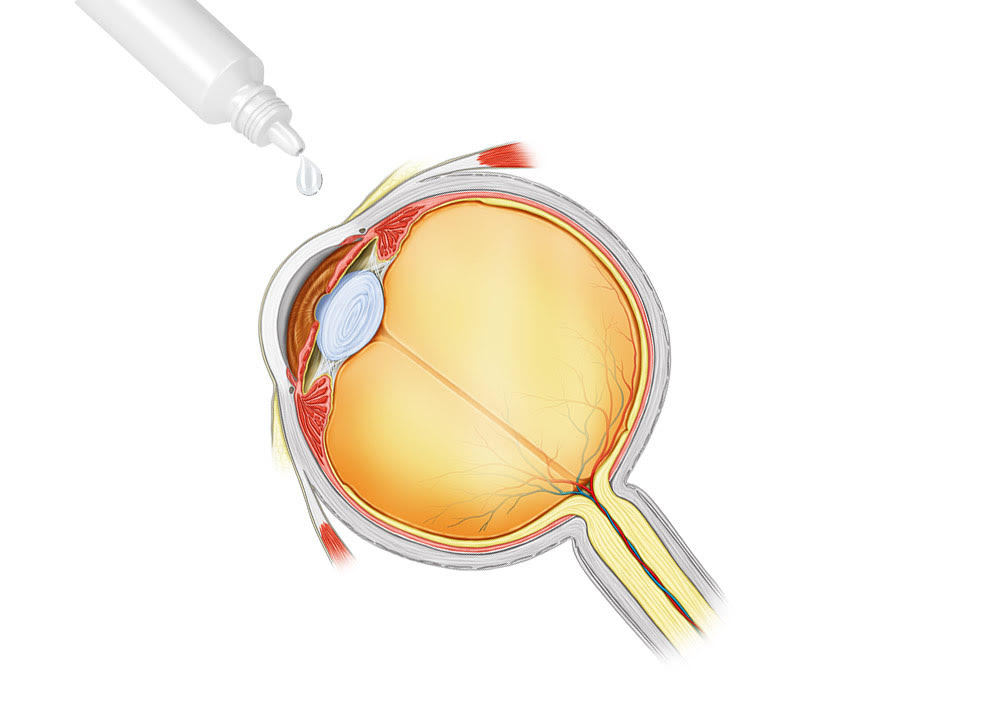
Topical TTHX1114
Product Development Status
TTHX1114 – eFGF1
Program
Endothelial
Indication
Product
Discovery
Pre-Clinical
Clinical
Market
*Resolution of visual acuity associated with corneal edema
Epithelial
Discovery
Pre-Clinical
Clinical
Market
Endothelial Regeneration Program
Intracameral Injection TTHX1114
Intracameral injection involves the injection of a small amount of TTHX1114 into the eye directly behind the cornea using a very small needle.
Clinical Need
Corneal endothelial cells (which line the interior of the cornea) are critical to maintaining the cornea in its appropriate state of hydration, enabling it to remain clear and function properly. A progressive degree of endothelial cell loss occurs naturally as a result of aging. However, surgically induced trauma to endothelial cells or accelerated loss or dysfunction of corneal endothelial cells from a variety of causes (corneal endothelial dystrophy) disrupts the hydration state of the cornea and can lead to corneal edema, corneal clouding, and diminished vision.
There is currently no treatment for endothelial damage nor a cure for corneal endothelial dystrophies (CED). Alternatives are symptomatic treatments, and, for advanced disease, corneal transplantation. Corneal transplants are effective but require life-long steroid treatment to prevent rejection and they are only used when vision is significantly impacted.
Each year in the United States there are over four million cataract surgeries and as many as 7% of patients may be at risk for serious surgically induced endothelial damage which may lead to prolonged edema that leads to sub-optimal visual recovery or in serious cases may require a corneal transplant.
CED (accelerated loss or dysfunction of some cells) affects 4% of the population, primarily over the age of 40, in the U.S. and E.U. (12 million in the U.S.), encompassing a number of disorders with a spectrum of severity. CED is the leading cause of corneal transplant surgery.
Development
The Trefoil product is designed to restore vision loss due to CED by stimulating the inherent regenerative properties of naturally occurring endothelial cells. TTHX1114 has shown great potential to:
- Improve vision, reduce corneal edema and accelerate recovery from both surgical endothelial damage and endothelial dystrophies
- Protect corneal endothelial cells from damage and stimulate endothelial proliferation and function
Trefoil has generated extensive non-clinical data in support of TTHX1114 use in FECD and other corneal endothelial dystrophies. These studies include in-vitro healing acceleration studies; animal models demonstrating accelerated healing and reduction in corneal opacity with TTHX1114 treatment; and in-vitro studies of normal and FECD human corneas demonstrating that TTHX1114 stimulates corneal endothelial cell protection, proliferation and migration.
The company has completed a Phase 2 clinical trial to assess TTHX1114’s potential to enhance corneal recovery and improve visual acuity in FECD patients undergoing a procedure to remove unhealthy corneal endothelial cells and guttae (collagen bodies produced by stressed corneal endothelial cells) in a small central area of the cornea. The procedure, called Descemetorhexis without Endothelial Keratoplasty (DWEK) or Descemet Stripping Only (DSO), does not include transplant of donor tissue and was recently developed as an alternative to corneal transplant.
In this study, 46% of the subjects received concomitant cataract surgery and these patients showed the same accelerated recovery of vision as those only undergoing the DSO procedure. This research supports the initiation of a further Phase 2 study at the end of 2023 to evaluate the potential of TTHX1114 to mitigate post-surgical edema experienced by patients with corneal endothelial risk factors.
Epithelial Damage Program
Topical Application TTHX1114
Clinical Need
Various diseases and conditions can affect the corneal epithelium, leading to vision impairment, ocular discomfort, and, in severe cases, blindness. In most of these cases, patients are only able to address the ocular manifestations of the underlying disease with palliative symptomatic treatments.
Sjögren's syndrome affects the ocular surface and the lacrimal glands, resulting in severe dry eye symptoms. With reduced tear production and compromised ocular surface protection the corneal epithelium becomes vulnerable to damage, infection, and chronic inflammation, potentially causing corneal thinning, scarring, and vision loss.
Herpetic keratopathy is a condition in which an estimated 500,000 patients annually suffer corneal complications of herpes virus reactivation in the US. Globally, 10 % of all blindness (four million people) is attributable to herpes infections.
There is a significant clinical need to reduce the duration of corneal ulcers during an activation event and reduce pain and inflammation. There are currently no treatments that directly address corneal ulcers related to herpes infection. Current treatments aim at reducing viral load with antiviral drugs, or reducing inflammation with corticosteroids. Severe cases are sometimes treated with surgery or mechanical barriers.
Neurotrophic keratitis can result from a variety of etiologies, including herpetic infection, surgery, and systemic diseases such as diabetes. The loss of corneal sensation leads to a reduction in tear production, blink reflex, and epithelial cell turnover, predisposing the cornea to breakdown, persistent epithelial defects, and, ultimately, ulceration and perforation. Standard treatments for epithelial defects, such as lubricants, bandage contact lenses, and tarsorrhaphy, may be insufficient for promoting healing.
Development
Trefoil is developing a topical (eye drop) version of TTHX1114 for a number of conditions in which the epithelial surface of the cornea has been compromised. The drug is expected to accelerate corneal wound healing, stimulate epithelial cell growth and reduce complications such as pain and inflammation. Topical TTHX1114 has potential to complement the use of other treatments for the underlying infection, such as antiviral drugs and surgery.
In preclinical studies, TTHX1114 significantly reduced corneal damage in a chemical injury model in studies supported by a grant from the U.S. Department of Defense. Trefoil, in conjunction with the University of California, Irvine, has also completed an animal model study in herpetic keratopathy. A Phase 1 safety study has been completed and plans are in place for initiation of a Phase 2 clinical trial in early 2024.
Future Opportunities
Corneal ulcers can arise from trauma, burns, chemical injury and in association with a variety of other diseases. These disorders all manifest in epithelial compromise or damage and represent future opportunities for topical TTHX1114 to meet important clinical needs.

